structure of the Grignard reagents In the category gold discovery more articles and learn more information about structure of the Grignard reagents Reviews Price Specifications Features Image manuals videos Accessories All this in metal detectors for gold.
When adding a solution of al keel halogen ide in dry diethyl ether (C2H5) 2O to magnesium metal with stirring vigorous reaction occurs; the solution becomes cloudy, begins to boil and the metal magnesium fades. The resulting solution is called Grignard reagents named Victor Grignard (University of Lyon), who in 1912 received a Nobel Prize discovery. This is one of the most useful and multilateral reagents known to organic chemists.
Grignard reagents has the formula and common name RMgX alkyl magnesium halide. It is believed that a carbon – magnesium is covalent, but strongly polar; Feedback magnesium – essentially halogen ion.
In fact, the structure of the Grignard reagents is much more complicated than the one given; for some Grignard reagents may not exist molecules RMgX; However for convenience, this formula is used organic chemists.
Since magnesium is bound to the same carbon atom to which had been bound halogen, an alkyl group during the synthesis of the reagent remains unchanged. Thus, of the n-propyl chloride formed n-propyl magnesium chloride, isopropyl chloride and from chloride.
Using the Grignard reagents due to its high reactivity. It reacts with various inorganic compounds, including water, carbon dioxide, oxygen, and with a large number of organic compounds; In many cases, such a reaction is the best route for the synthesis of organic compounds of a certain class.
Reaction with water, leading to the formation of an alkane, characterizes the ratio of the Grignard reagents to acids. As already mentioned, a carbon – magnesium strongly polar or, in other words, is largely ionic. Consequently, the Grignard reagent can be considered a magnesium salt RMgX and very weak acid R-H. In reaction
weaker acid R – H is displaced from its salt stronger acid HOH.
Alkane – so weak acid, it is displaced from the Grignard compounds, which themselves usually considered very weak acids or even not regarded acids. Any compound containing a hydrogen attached to oxygen or nitrogen, is significantly more acidic than the alkane, and hence, it can decompose the Grignard reagents, for example ammonia or methanol. To obtain an alkane can use any acid, so it is natural that they take the most affordable and convenient – water.
structure of the Grignard reagents
- Digital Video Recording System
- fisher f 75
- Bank of Mexico coined a kilo gold coin
- If you plan to be rich, learn some rules
- ground penetrating radar units The IDS Opera
- Test del Garrett Ace 250 sulla battigia reviews
- أسعار اجهزة كشف المعادن غاريت
- Using a satellite survey of the research on the underground
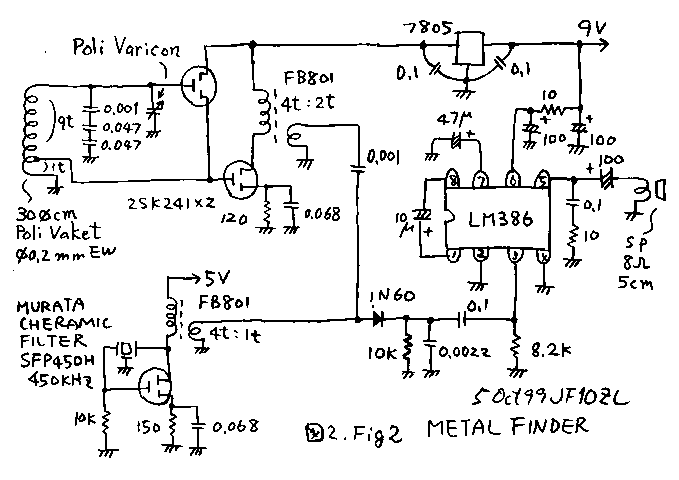
- Metal detector circuit diagram
- Bounty Hunter Fast Tracker Metal Detector